SAROGLITAZAR – FIRST NCE DISCOVERED AND DEVELOPED IN INDIA TO REACH THE MARKET
Zydus Cadila announced on June 5 2013 the approval by the Drug Controller General of India (DCGI) of Saroglitazar (ZYH1), or LipaglynTM. The drug has been approved for launch in India for the treatment of diabetic dyslipidemia or hypertriglyceridemia in patients with type II diabetes not controlled by statins alone [1] .
LipaglynTM is the first glitazar to be approved in the world, and is the first NCE discovered and developed indigenously by an Indian company. The drug originates from a research program initiated at Zydus Cadila in 2000, and an IND submission in 2004 after extensive structure-activity relationship studies and preclinical characterization.
The compound belongs to the class of ‘glitazars’, dual peroxisome proliferator-activated receptor (PPAR) agonists with affinity towards both PPARα and PPARγ [2] [3]. According to Zydus Cadila [1], Saroglitazar has a predominant affinity for the PPARα isoform, and a moderate affinity for PPARγ, and has shown beneficial effects on lipids and glycemic controls without side effects. At a dose of 4 mg once daily, it reduces triglycerides and LDL cholesterol, it increases HDL cholesterol, and also shows a reduction in Fasting Plasma Glucose and glycosylated hemoglobin.
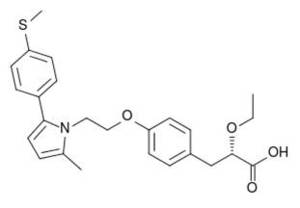
Chemical structure of Saroglitazar
|
Saroglitazar, or (2S)-2-ethoxy-3-[4-(2-(2-methyl-5-[4-(methylsulfanyl)phenyl]-1H-pyrrol-1-yl)ethoxy) phenyl]propanoic acid, has been described in patent application WO 03/009841 A1, with a priority date of July 26, 2001, for its Indian patent application (711/MUM/2001).
|
The class of ‘glitazars’ also includes compounds such as:
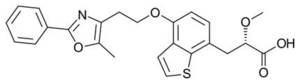
- Hoffmann-La Roche’s Aleglitazar (R1439), currently in Ph III trials [4]
|
|
|
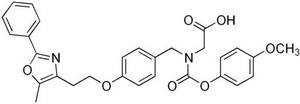
- Bristol-Myers Squibb’s Muraglitazar (BMS-298585), which had completed Ph III clinical trials, but was discontinued in 2006
|
|
|
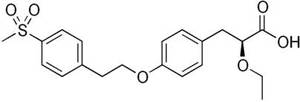
- AstraZeneca’s Tesaglitazar, also discontinued after Ph III trials in 2007
|
|
|
With 20 discovery research programs and a biosimilar pipeline, Zydus Cadila’s current NCE R&D pipeline, largely focused on diabetes and metabolic disorders, includes six compounds in Ph I and Ph II clinical stages [5].
Table: Zydus Cadila NCE pipeline
| Project |
Target |
Indication |
Status |
| ZYH7 |
PPARα agonist |
Dyslipidemia |
Ph II |
| ZYD1 |
GLP-1 agonist |
Diabetes, obesity |
Ph I |
| ZYOG1 |
Oral GLP-1 agonist |
Diabetes, obesity |
Ph I |
| ZYGK1 |
Glucokinase activator |
Diabetes |
Ph I |
| ZYG19 |
GPR-119 agonist |
Diabetes |
Ph I |
| ZYPH0907 |
Oral PTH agonist |
Osteoporosis |
Ph I |
|
Additional Zydus Cadila compounds had progressed into clinical studies, but have been abandoned, such as:
- ZYI1, a multi-modal compound for the treatment of pain,
- ZYO1, a CB-1 antagonist for the treatment of obesity and diabetes
- ZYH2, another dual PPARα/PPARγ agonist for diabetes
- ZYT1, a Thyroid hormone receptor beta agonist for the treatment of dyslipidemia
Zydus Cadila invests over 7% of its turnover in research. The group employs over 15,000 people worldwide, and at the group’s state-of-the-art research arm, the Zydus Research Centre, located in Ahmedabad, over 400 research scientists are engaged in NCE research alone [1].
|
Bibliography
[1] Zydus Cadila, "Zydus pioneers a breakthrough with LIPAGLYN, India’s first NCE to reach the market," 5 June 2013. [Online].
Available: http://www.zyduscadila.com/press/PressNote05-06-13.pdf.
[2] "PPAR agonist," [Online].
Available: http://en.wikipedia.org/wiki/PPAR_agonist.
[3] D. K. Nevin, D. Fayne and D. G. Lloyd, "Rational targeting of peroxysome proliferating activated receptor subtypes," Current Medicinal Chemistry, vol. 18, no. 36, pp. 5598-5623, 2011.
[4] Hoffmann-La Roche, 2013. [Online].
Available: http://www.roche.com/research_and_development/r_d_overview/pharmaceuticals/r_d_metabolism.htm.
[5] Zydus Cadila, 2013. [Online]. Available: http://www.zyduscadila.com/discovery.html.
|
|
|